

When the rays were bent by a magnetic field, they infiltrate the cylinders through the slits. He traced the path of the rays using the fluorescence on a squared screen in the jar. The rays would not enter the cylinders unless deflected by a magnetic field. When a high potential difference was applied between the cathode (A in the diagram) and anode (B in the diagram), cathode rays, which were produced in the left tube, emitted from the cathode and entered into the main bell jar. The outer cylinder was grounded while inner was attached to an electrometer to detect any electric current as shown in the figure below. The cylinders were coaxial placed and insulated from each other. The experiment apparatus consisted of two metal cylinders. In the final experiment, he succeeded in measuring mass to charge ratio. In the first, the magnetic effect on cathode rays was studied while in the second, the rays were deflected by an electric field. His entire works can be divided into three different experiments. He concluded that the rays were comprised of particles. The immaterial nature and the aetherial hypothesis of cathode rays were proved wrong by J. According to some, the rays are due to some process in the aether. Many diverse opinions were held on these rays.
#JJ THOMSON CATHODE RAY EXPERIMENT RESULTS SERIES#

The cathode-ray tube (CRT) is a hollow glass tube. These rays travel in straight lines and can be deflected by electric and magnetic field.

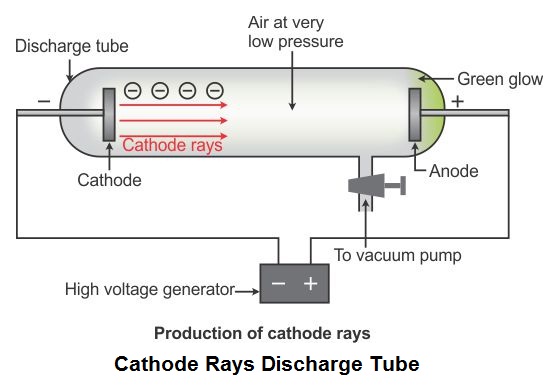
What are cathode rays? Cathode rays are streams of electrons emitted from the cathode (the electrode connected to the negative terminal of a battery). His experimental results were further investigated by Rutherford and Bohr, which further provided important insights into the atomic world.īefore directly jumping Thomson's findings, let us understand some basic knowledge on cathode rays and the cathode-ray tube. However, Thomson's contributions remain more significant than the rest. Thomson was not the only one working on cathode rays, but several other players like Julius Plücker, Johann Wilhelm Hittorf, William Crookes, Philipp Lenard had contributed or were busy studying it. The apparatus of his experiment is called the cathode-ray tube (CRT). These particles later were named electrons. In 1897, he showed that cathode rays were composed of very small negatively charged particles. He was well-known for the discovery of the electron. Sir Joseph John Thomson was a British physicist and Nobel Laureate.


 0 kommentar(er)
0 kommentar(er)
